Kullanıcı:Brcan/Denem/Tek başına çeviriler/Çalışma 3


Bitkilerin evrimi, en ilkel alglerden çok hücreli deniz ve tatlı su alglerine; kara yosunlarından açık ve kapalı tohumlulara kadar karmaşık bir süreçte gerçekleşmiştir. Birçok erken grup gelişmeye devam ederken, denizlerdeki kırmızı ve yeşil alglerde olduğu gibi, yeni türemiş gruplar, eski baskın grupların yerini almaya başladı. Açık tohumlulardan kapalı tohumlularin türemesi bunun bir örneğidir.[6]:498
Siyanobakterilerin ve çok hücreli fotosentetik ökaryotların 1 milyar yıl önce tatli su topluluklarında yaşadığına[7] ve karmaşık, çok hücreli fotosentetik organizmaların Kambriyen öncesinde, 850 milyon yıl önce karada yaşadıklarına dair güçlü kanıtlar vardır.[8]
Embriyofit kata bitkileri ilk Orta Ordovisyen'de (~470 milyon yıl önce) ortaya çıkmışlardı ve Orta Devoniyen'de (~390 milyon yıl önce), kara bitkilerinde var olan kök ve yapraklar dahil birçok özellik oluşmuştu. Geç Devoniyen'de (~370 milyon yıl önce) Archaeopteris gibi bazı pteridofitler oluşmuşu; ikincil damar dokuları sayesinde odun üretilebiliyordu ve uzun ağaç ormanları yetişiyordu. Ayrıca Geç Devoniyen'de, bir erken eğreltiotu olan Elkinsia'da tohum evrimleşmiştir.[9] Evrimsel gelişim Fanerozoik Devir'in geri kalanı boyunca devam etti ve hâlâ devam etmektedir. Bitki gruplarının çoğu Permo-Trias'tan sonra yok oldu, buna rağmen bu toplulukların yapıları değişime uğradı. Bu, Triyas Devri'nde (~200 milyon yıl önce) çiçekli bitkilerin ortaya çıkmasına ve sonrasında Kretase ve Palosen'de çeşitlenmesine zemin hazırlamış olabilir. Evrimleşmiş en son büyük bitki grubu, Orta Palosen'de (~40 milyon yıl önce) önem kazanan çimlerdi. Çimler, diğer gruplarda olduğu gibi, düşük karbondioksit ve ısıda, kuru koşullarda hayatta kalmak için son 10 milyon yılda yeni metabolizmik mekanizmalar geliştirdiler.
Bitkilerin morfolojik evrimi[değiştir | kaynağı değiştir]
Yapraklar[değiştir | kaynağı değiştir]


Yapraklar, modern bitkinin birincil fotosentetik organıdır. Yaprakların kökeni, atmosferdeki karbondioksitin Devoniyen Devri sırasında düşmesine dayanır; bu dönemde fotosentez yapmak için karbondioksitin alınma verimliliğinin önemi artmıştır.[10][11]
Yaprakların birden fazla kez evrim geçirdiği kesindir. Yapılarına göre ikiye ayrılırlar: karmaşık damar yapısından yoksun olan ve kökeninin dikenli çıkıntılara dayandığı düşünülen mikropiller ve geniş, karmaşık damar yapısına sahip olan ve şube gruplarındaki değişiklikler sayesinde geliştiği düşünülen makropiller. Bu yapıların birbirinden bağımsız olarak geliştiği önerilmiştir.[12] Megapiller, Walter Zimmerman'in telomer teorisine göre,[13] üç boyutlu budaklı yapılar gösteren bitkilerden evrimleşmiştir; bu üç nedenin etkisiyle gerçekleşti: tipik bir yaprağın yatay şekline sahip olmasını sağlayan aşma, düz bir yapının oluşmasını sağlayan düzleşme, dalların birleşmesini sağlayan ağlaşma veya füzyon, böylece uygun bir yaprak tabakası oluştu. Bütün bu aşamalar birden fazla kez tekrarlandı.[14]
Genel kanı, telomer teorisinin fosiller ile iyi bir şekilde desteklendiği yönündedir. Yine de Wolfgang Hagemann, morfolojik ve ekolojik nedenleri inceledi ve alternatif bir teori önerdi.[15][16] Buna karşın telomer teorisine göre, en ilkel kara bitkilerinin radyal simetrik eksenlerinin üç boyutlu damar sistemleri vardı (telomerler); Hagemann ise en ilkel kara bitkilerinin eksensiz, yaprak benzeri, düz olduğuna.ve koyunotu veya eğreltiotundaki gibi bir şekle sahip olduğunu iddia etti. Ona göre sap ve kökler yeni organlar olarak evrimleşmişlerdi.
Rolf Sattler proposed an overarching process-oriented view that leaves some limited room for both the telome theory and Hagemann's alternative and in addition takes into consideration the whole continuum between dorsiventral (flat) and radial (cylindrical) structures that can be found in fossil and living land plants.[17][18] Bu göŕüş moleküler genetik araştırmalarıyla desteklendi. James (2009),[19] "radyal [sap gibi eksenlerin karekteristliği] ve dorsiventral [yaprakların karekteristliği] genel olarak kabül görmüştür söylemlerinin sürekli bir spektrumun iki ucu olduğuna ve bunu KNOX gen aktarımının basit bir zamanlaması olduğuna" karar vermiştir.
Bitkiler evrimleşmeden önce, bitkilerin kökleri üzerinde fotosentetik aparatları vardı. Bugünün megapil yaprakları muhtemelen 360 milyon yıl önce, basit yapraksız bitkilerin dünyayı kolonize ettiği Erken Devoniyen'den 40 milyon yıl sonra, yaygınlaşmıştı. Bu, Geç Paleozoik dönemde, atmosferik karbondioksitte düşüşe neden oldu.This spread has been linked to the fall in the atmospheric carbon dioxide concentrations in the Late Paleozoic era associated with a rise in density of stomata on leaf surface.[10] This would have resulted in greater transpiration rates and gas exchange, but especially at high CO2 concentrations, large leaves with fewer stomata would have heated to lethal temperatures in full sunlight. Increasing the stomatal density allowed for a better-cooled leaf, thus making its spread feasible, but increased CO2 uptake at the expense of decreased water use efficiency.[11][20]
The rhyniophytes of the Rhynie chert consisted of nothing more than slender, unornamented axes. The early to middle Devonian trimerophytes may be considered leafy. This group of vascular plants are recognisable by their masses of terminal sporangia, which adorn the ends of axes which may bifurcate or trifurcate.[21] Some organisms, such as Psilophyton, bore enations. These are small, spiny outgrowths of the stem, lacking their own vascular supply.
The zosterophylls were already important in the late Silurian, much earlier than any rhyniophytes of comparable complexity.[22] This group, recognisable by their kidney-shaped sporangia which grew on short lateral branches close to the main axes, sometimes branched in a distinctive H-shape.[21] Many zosterophylls bore pronounced spines on their axes[kaynak belirtilmeli] but none of these had a vascular trace. The first evidence of vascularised enations occurs in a fossil clubmoss known as Baragwanathia that had already appeared in the fossil record in the Late Silurian.[23] In this organism, these leaf traces continue into the leaf to form their mid-vein.[24] One theory, the "enation theory", holds that the microphyllous leaves of clubmosses developed by outgrowths of the protostele connecting with existing enations[21] The leaves of the Rhynie genus Asteroxylon, which was preserved in the Rhynie chert almost 20 Million years later than Baragwanathia had a primitive vascular supply – in the form of leaf traces departing from the central protostele towards each individual "leaf".[25] Asteroxylon and Baragwanathia are widely regarded as primitive lycopods,[21] a group still extant today, represented by the quillworts, the spikemosses and the club mosses. Lycopods bear distinctive microphylls, defined as leaves with a single vascular trace. Microphylls could grow to some size, those of Lepidodendrales reaching over a meter in length, but almost all just bear the one vascular bundle. An exception is the rare branching in some Selaginella species.
The more familiar leaves, megaphylls, are thought to have originated four times independently, in the ferns, horsetails, progymnosperms and seed plants.[26] They appear to have originated by modifying dichotomising branches, which first overlapped (or "overtopped") one another, became flattened or planated and eventually developed "webbing" and evolved gradually into more leaf-like structures.[24] Megaphylls, by Zimmerman's telome theory, are composed of a group of webbed branches[24] and hence the "leaf gap" left where the leaf's vascular bundle leaves that of the main branch resembles two axes splitting.[24] In each of the four groups to evolve megaphylls, their leaves first evolved during the Late Devonian to Early Carboniferous, diversifying rapidly until the designs settled down in the mid Carboniferous.[26]
The cessation of further diversification can be attributed to developmental constraints,[26] but why did it take so long for leaves to evolve in the first place? Plants had been on the land for at least 50 million years before megaphylls became significant. However, small, rare mesophylls are known from the early Devonian genus Eophyllophyton – so development could not have been a barrier to their appearance.[27] The best explanation so far incorporates observations that atmospheric Şablon:Co2 was declining rapidly during this time – falling by around 90% during the Devonian.[28] This required an increase in stomatal density by 100 times to maintain rates of photosynthesis. When stomata open to allow water to evaporate from leaves it has a cooling effect, resulting from the loss of latent heat of evaporation. It appears that the low stomatal density in the early Devonian meant that evaporation and evaporative cooling were limited, and that leaves would have overheated if they grew to any size. The stomatal density could not increase, as the primitive steles and limited root systems would not be able to supply water quickly enough to match the rate of transpiration.[11] Clearly, leaves are not always beneficial, as illustrated by the frequent occurrence of secondary loss of leaves, famously exemplified by cacti and the "whisk fern" Psilotum.
Secondary evolution can also disguise the true evolutionary origin of some leaves. Some genera of ferns display complex leaves which are attached to the pseudostele by an outgrowth of the vascular bundle, leaving no leaf gap.[24] Further, horsetail (Equisetum) leaves bear only a single vein, and appear to be microphyllous; however, both the fossil record and molecular evidence indicate that their forebears bore leaves with complex venation, and the current state is a result of secondary simplification.[29]
Deciduous trees deal with another disadvantage to having leaves. The popular belief that plants shed their leaves when the days get too short is misguided; evergreens prospered in the Arctic circle during the most recent greenhouse earth.[30] The generally accepted reason for shedding leaves during winter is to cope with the weather – the force of wind and weight of snow are much more comfortably weathered without leaves to increase surface area. Seasonal leaf loss has evolved independently several times and is exhibited in the ginkgoales, some pinophyta and certain angiosperms.[31] Leaf loss may also have arisen as a response to pressure from insects; it may have been less costly to lose leaves entirely during the winter or dry season than to continue investing resources in their repair.[32]
Factors influencing leaf architectures[değiştir | kaynağı değiştir]
Various physical and physiological factors such as light intensity, humidity, temperature, wind speeds etc. have influenced evolution of leaf shape and size. High trees rarely have large leaves, because they are damaged by high winds. Similarly, trees that grow in temperate or taiga regions have pointed leaves,[kaynak belirtilmeli] presumably to prevent nucleation of ice onto the leaf surface and reduce water loss due to transpiration. Herbivory, by mammals and insects, has been a driving force in leaf evolution. An example is that plants of the New Zealand genus Aciphylla have spines on their laminas, which probably functioned to discourage the extinct Moas from feeding on them. Other members of Aciphylla, which did not co-exist with the moas, do not have these spines.[33]
At the genetic level, developmental studies have shown that repression of KNOX genes is required for initiation of the leaf primordium. This is brought about by ARP genes, which encode transcription factors. Repression of KNOX genes in leaf primordia seems to be quite conserved, while expression of KNOX genes in leaves produces complex leaves. The ARP function appears to have arisen early in vascular plant evolution, because members of the primitive group Lycophytes also have a functionally similar gene.[34] Other players that have a conserved role in defining leaf primordia are the phytohormones auxin, gibberelin and cytokinin.

The arrangement of leaves or phyllotaxy on the plant body can maximally harvest light and might be expected to be genetically robust. However, in maize, a mutation in only one gene called ABPHYL (ABnormal PHYLlotaxy) is enough to change the phyllotaxy of the leaves, implying that mutational adjustment of a single locus on the genome is enough to generate diversity.[35]
Once the leaf primordial cells are established from the SAM cells, the new axes for leaf growth are defined, among them being the abaxial-adaxial (lower-upper surface) axes. The genes involved in defining this, and the other axes seem to be more or less conserved among higher plants. Proteins of the HD-ZIPIII family have been implicated in defining the adaxial identity. These proteins deviate some cells in the leaf primordium from the default abaxial state, and make them adaxial. In early plants with leaves, the leaves probably just had one type of surface — the abaxial one, the underside of today's leaves. The definition of the adaxial identity occurred some 200 million years after the abaxial identity was established.[36]
How the wide variety of observed plant leaf morphology is generated is a subject of intense research. Some common themes have emerged. One of the most significant is the involvement of KNOX genes in generating compound leaves, as in the tomato (see above). But, this is not universal. For example, the pea uses a different mechanism for doing the same thing.[37][38] Mutations in genes affecting leaf curvature can also change leaf form, by changing the leaf from flat, to a crinkly shape,[39] like the shape of cabbage leaves. There also exist different morphogen gradients in a developing leaf which define the leaf's axis and may also affect the leaf form. Another class of regulators of leaf development are the microRNAs.[40][41]
Roots[değiştir | kaynağı değiştir]
Roots are important to plants for two main reasons: Firstly, they provide anchorage to the substrate; more importantly, they provide a source of water and nutrients from the soil. Roots allowed plants to grow taller and faster.
The evolution of roots had consequences on a global scale. By disturbing the soil and promoting its acidification (by taking up nutrients such as nitrate and phosphate[42]), they enabled it to weather more deeply, injecting carbon compounds deeper into soils[43] with huge implications for climate.[44] These effects may have been so profound they led to a mass extinction.[45]
While there are traces of root-like impressions in fossil soils in the Late Silurian,[46] body fossils show the earliest plants to be devoid of roots. Many had prostrate branches that sprawled along the ground, with upright axes or thalli dotted here and there, and some even had non-photosynthetic subterranean branches which lacked stomata. The distinction between root and specialised branch is developmental.[kaynak belirtilmeli] differing in their branching pattern and in possession of a root cap.[47] So while Siluro-Devonian plants such as Rhynia and Horneophyton possessed the physiological equivalent of roots,[48][49] roots – defined as organs differentiated from stems – did not arrive until later.[47] Unfortunately, roots are rarely preserved in the fossil record, and our understanding of their evolutionary origin is sparse.[47]
Rhizoids – small structures performing the same role as roots, usually a cell in diameter – probably evolved very early, perhaps even before plants colonised the land; they are recognised in the Characeae, an algal sister group to land plants.[47] That said, rhizoids probably evolved more than once; the rhizines of lichens, for example, perform a similar role. Even some animals (Lamellibrachia) have root-like structures.[47] Rhizoids are clearly visible in the Rhynie chert fossils, and were present in most of the earliest vascular plants, and on this basis seem to have presaged true plant roots.[50]
More advanced structures are common in the Rhynie chert, and many other fossils of comparable early Devonian age bear structures that look like, and acted like, roots.[47] The rhyniophytes bore fine rhizoids, and the trimerophytes and herbaceous lycopods of the chert bore root-like structure penetrating a few centimetres into the soil.[51] However, none of these fossils display all the features borne by modern roots,[47] with the exception of Asteroxylon, which has recently been recognized as bearing roots that evolved independently from those of extant vascular plants.[52] Roots and root-like structures became increasingly common and deeper penetrating during the Devonian, with lycopod trees forming roots around 20 cm long during the Eifelian and Givetian. These were joined by progymnosperms, which rooted up to about a metre deep, during the ensuing Frasnian stage.[51] True gymnosperms and zygopterid ferns also formed shallow rooting systems during the Famennian.[51]
The rhizophores of the lycopods provide a slightly different approach to rooting. They were equivalent to stems, with organs equivalent to leaves performing the role of rootlets.[47] A similar construction is observed in the extant lycopod Isoetes, and this appears to be evidence that roots evolved independently at least twice, in the lycophytes and other plants,[47] a proposition supported by studies showing that roots are initiated and their growth promoted by different mechanisms in lycophytes and euphyllophytes.[53]
A vascular system is indispensable to rooted plants, as non-photosynthesising roots need a supply of sugars, and a vascular system is required to transport water and nutrients from the roots to the rest of the plant.[54] Rooted plantsŞablon:Which are little more advanced than their Silurian forebears, without a dedicated root system; however, the flat-lying axes can be clearly seen to have growths similar to the rhizoids of bryophytes today.[55]
By the Middle to Late Devonian, most groups of plants had independently developed a rooting system of some nature.[55] As roots became larger, they could support larger trees, and the soil was weathered to a greater depth.[45] This deeper weathering had effects not only on the aforementioned drawdown of Şablon:Co2, but also opened up new habitats for colonisation by fungi and animals.[51]
Roots today have developed to the physical limits. They penetrate as much as 60 metres of soil to tap the water table.[56] The narrowest roots are a mere 40 μm in diameter, and could not physically transport water if they were any narrower.[47] The earliest fossil roots recovered, by contrast, narrowed from 3 mm to under 700 μm in diameter; of course, taphonomy is the ultimate control of what thickness can be seen.[47]
Tree form[değiştir | kaynağı değiştir]


The early Devonian landscape was devoid of vegetation taller than waist height. Greater height provided a competitive advantage in the harvesting of sunlight for photosynthesis, overshadowing of competitors and in spore distribution, as spores (and later, seeds) could be blown for greater distances if they started higher. An effective vascular system was required in order to achieve greater heights. To attain arborescence, plants had to develop woody tissue that provided both support and water transport, and thus needed to evolve the capacity for secondary growth. The stele of plants undergoing secondary growth is surrounded by a vascular cambium, a ring of meristematic cells which produces more xylem on the inside and phloem on the outside. Since xylem cells comprise dead, lignified tissue, subsequent rings of xylem are added to those already present, forming wood. Fossils of plants from the early Devonian show that a simple form of wood first appeared at least 400 million years ago, at a time when all land plants were small and herbaceous.[57] Because wood evolved long before shrubs and trees, it is likely that its original purpose was for water transport, and that it was only used for mechanical support later.[58]
The first plants to develop secondary growth and a woody habit, were apparently the ferns, and as early as the Middle Devonian one species, Wattieza, had already reached heights of 8 m and a tree-like habit.[59]
Other clades did not take long to develop a tree-like stature. The Late Devonian Archaeopteris, a precursor to gymnosperms which evolved from the trimerophytes,[60] reached 30 m in height. The progymnosperms were the first plants to develop true wood, grown from a bifacial cambium. The first appearance of one of them, Rellimia, was in the Middle Devonian.[61] True wood is only thought to have evolved once, giving rise to the concept of a "lignophyte" clade.[kaynak belirtilmeli]
Archaeopteris forests were soon supplemented by arborescent lycopods, in the form of Lepidodendrales, which exceeded 50m in height and 2m across at the base. These arborescent lycopods rose to dominate Late Devonian and Carboniferous forests that gave rise to coal deposits.[62] Lepidodendrales differ from modern trees in exhibiting determinate growth: after building up a reserve of nutrients at a lower height, the plants would "bolt" as a single trunk to a genetically determined height, branch at that level, spread their spores and die.[63] They consisted of "cheap" wood to allow their rapid growth, with at least half of their stems comprising a pith-filled cavity.[21] Their wood was also generated by a unifacial vascular cambium – it did not produce new phloem, meaning that the trunks could not grow wider over time.Şablon:Verify source
The horsetail Calamites appeared in the Carboniferous. Unlike the modern horsetail Equisetum, Calamites had a unifacial vascular cambium, allowing them to develop wood and grow to heights in excess of 10 m and to branch repeatedly.
While the form of early trees was similar to that of today's, the Spermatophytes or seed plants, the group that contain all modern trees, had yet to evolve. The dominant tree groups today are all seed plants, the gymnosperms, which include the coniferous trees, and the angiosperms, which contain all fruiting and flowering trees. No free-sporing trees like Archaeopteris exist in the extant flora. It was long thought that the angiosperms arose from within the gymnosperms, but recent molecular evidence suggests that their living representatives form two distinct groups.[64][65][66] The molecular data has yet to be fully reconciled with morphological data,[67][68][69] but it is becoming accepted that the morphological support for paraphyly is not especially strong.[70] This would lead to the conclusion that both groups arose from within the pteridosperms, probably as early as the Permian.[70]
The angiosperms and their ancestors played a very small role until they diversified during the Cretaceous. They started out as small, damp-loving organisms in the understorey, and have been diversifying ever since the midŞablon:Verify source-Cretaceous, to become the dominant member of non-boreal forests today.
Seeds[değiştir | kaynağı değiştir]


Early land plants reproduced in the fashion of ferns: spores germinated into small gametophytes, which produced eggs and/or sperm. These sperm would swim across moist soils to find the female organs (archegonia) on the same or another gametophyte, where they would fuse with an egg to produce an embryo, which would germinate into a sporophyte.[51]
Heterosporic plants, as their name suggests, bear spores of two sizes – microspores and megaspores. These would germinate to form microgametophytes and megagametophytes, respectively. This system paved the way for ovules and seeds: taken to the extreme, the megasporangia could bear only a single megaspore tetrad, and to complete the transition to true ovules, three of the megaspores in the original tetrad could be aborted, leaving one megaspore per megasporangium.
The transition to ovules continued with this megaspore being "boxed in" to its sporangium while it germinated. Then, the megagametophyte was contained within a waterproof integument, which enclosed the seed. The pollen grain, which contained a microgametophyte germinated from a microspore , was employed for dispersal of the male gamete, only releasing its desiccation-prone flagellate sperm when it reached a receptive megagametophyte.[21]
Lycopods and sphenopsids got a fair way down the path to the seed habit without ever crossing the threshold. Fossil lycopod megaspores reaching 1 cm in diameter, and surrounded by vegetative tissue, are known (Lepidocarpon, Achlamydocarpon);– these even germinated into a megagametophyte in situ. However, they fell short of being ovules, since the nucellus, an inner spore-covering layer, does not completely enclose the spore. A very small slit (micropyle) remains, meaning that the megasporangium is still exposed to the atmosphere. This has two consequences – firstly, it means it is not fully resistant to desiccation, and secondly, sperm do not have to "burrow" to access the archegonia of the megaspore.[21]
A Middle Devonian precursor to seed plants from Belgium has been identified predating the earliest seed plants by about 20 million years. Runcaria, small and radially symmetrical, is an integumented megasporangium surrounded by a cupule. The megasporangium bears an unopened distal extension protruding above the multilobed integument. It is suspected that the extension was involved in anemophilous pollination. Runcaria sheds new light on the sequence of character acquisition leading to the seed. Runcaria has all of the qualities of seed plants except for a solid seed coat and a system to guide the pollen to the ovule.[71]
The first spermatophytes (literally: "seed plants") – that is, the first plants to bear true seeds – are called pteridosperms: literally, "seed ferns", so called because their foliage consisted of fern-like fronds, although they were not closely related to ferns. The oldest fossil evidence of seed plants is of Late Devonian age, and they appear to have evolved out of an earlier group known as the progymnosperms. These early seed plants ranged from trees to small, rambling shrubs; like most early progymnosperms, they were woody plants with fern-like foliage. They all bore ovules, but no cones, fruit or similar. While it is difficult to track the early evolution of seeds, the lineage of the seed ferns may be traced from the simple trimerophytes through homosporous Aneurophytes.[21]
This seed model is shared by basically all gymnosperms (literally: "naked seeds"), most of which encase their seeds in a woody cone or fleshy aril (the yew, for example), but none of which fully enclose their seeds. The angiosperms ("vessel seeds") are the only group to fully enclose the seed, in a carpel.
Fully enclosed seeds opened up a new pathway for plants to follow: that of seed dormancy. The embryo, completely isolated from the external atmosphere and hence protected from desiccation, could survive some years of drought before germinating. Gymnosperm seeds from the Late Carboniferous have been found to contain embryos, suggesting a lengthy gap between fertilisation and germination.[72] This period is associated with the entry into a greenhouse earth period, with an associated increase in aridity. This suggests that dormancy arose as a response to drier climatic conditions, where it became advantageous to wait for a moist period before germinating.[72] This evolutionary breakthrough appears to have opened a floodgate: previously inhospitable areas, such as dry mountain slopes, could now be tolerated, and were soon covered by trees.[72]
Seeds offered further advantages to their bearers: they increased the success rate of fertilised gametophytes, and because a nutrient store could be "packaged" in with the embryo, the seeds could germinate rapidly in inhospitable environments, reaching a size where it could fend for itself more quickly.[51] For example, without an endosperm, seedlings growing in arid environments would not have the reserves to grow roots deep enough to reach the water table before they expired from dehydration.[51] Likewise, seeds germinating in a gloomy understory require an additional reserve of energy to quickly grow high enough to capture sufficient light for self-sustenance.[51] A combination of these advantages gave seed plants the ecological edge over the previously dominant genus Archaeopteris, thus increasing the biodiversity of early forests.[51]
Despite these advantages, it is common for fertilized ovules to fail to mature as seeds.[73] Also during seed dormancy (often associated with unpredictable and stressful conditions) DNA damage accumulates.[74][75][76] Thus DNA damage appears to be a basic problem for survival of seed plants, just as DNA damage is a major problem for life in general.[77]
Flowers[değiştir | kaynağı değiştir]

Flowers are modified leaves possessed only by the angiosperms, which are relatively late to appear in the fossil record. The group originated and diversified during the Early Cretaceous and became ecologically significant thereafter.[78] Flower-like structures first appear in the fossil records some ~130 mya, in the Cretaceous.[79] However, in 2018, scientists reported the finding of a fossil flower from about 180 million years ago, 50 million years earlier than thought earlier.[80] The interpretation has been however highly disputed.[81]
Colorful and/or pungent structures surround the cones of plants such as cycads and Gnetales, making a strict definition of the term "flower" elusive.[69]
The main function of a flower is reproduction, which, before the evolution of the flower and angiosperms, was the job of microsporophylls and megasporophylls. A flower can be considered a powerful evolutionary innovation, because its presence allowed the plant world to access new means and mechanisms for reproduction.
The flowering plants have long been assumed to have evolved from within the gymnosperms; according to the traditional morphological view, they are closely allied to the Gnetales. However, as noted above, recent molecular evidence is at odds with this hypothesis,[65][66] and further suggests that Gnetales are more closely related to some gymnosperm groups than angiosperms,[64] and that extant gymnosperms form a distinct clade to the angiosperms,[64][65][66] the two clades diverging some 300 milyon yıl önce.[82]
The relationship of stem groups to the angiosperms is important in determining the evolution of flowers. Stem groups provide an insight into the state of earlier "forks" on the path to the current state. Convergence increases the risk of misidentifying stem groups. Since the protection of the megagametophyte is evolutionarily desirable, probably many separate groups evolved protective encasements independently. In flowers, this protection takes the form of a carpel, evolved from a leaf and recruited into a protective role, shielding the ovules. These ovules are further protected by a double-walled integument.
Penetration of these protective layers needs something more than a free-floating microgametophyte. Angiosperms have pollen grains comprising just three cells. One cell is responsible for drilling down through the integuments, and creating a conduit for the two sperm cells to flow down. The megagametophyte has just seven cells; of these, one fuses with a sperm cell, forming the nucleus of the egg itself, and another joins with the other sperm, and dedicates itself to forming a nutrient-rich endosperm. The other cells take auxiliary roles.[kaynak belirtilmeli] This process of "double fertilisation" is unique and common to all angiosperms.

In the fossil record, there are three intriguing groups which bore flower-like structures. The first is the Permian pteridosperm Glossopteris, which already bore recurved leaves resembling carpels. The Mesozoic Caytonia is more flower-like still, with enclosed ovules – but only a single integument. Further, details of their pollen and stamens set them apart from true flowering plants.
The Bennettitales bore remarkably flower-like organs, protected by whorls of bracts which may have played a similar role to the petals and sepals of true flowers; however, these flower-like structures evolved independently, as the Bennettitales are more closely related to cycads and ginkgos than to the angiosperms.[83]
However, no true flowers are found in any groups save those extant today. Most morphological and molecular analyses place Amborella, the nymphaeales and Austrobaileyaceae in a basal clade called "ANA". This clade appear to have diverged in the early Cretaceous, around 130 milyon yıl önce – around the same time as the earliest fossil angiosperm,[84][85] and just after the first angiosperm-like pollen, 136 million years ago.[70] The magnoliids diverged soon after, and a rapid radiation had produced eudicots and monocots by 125 milyon yıl önce.[70] By the end of the Cretaceous Şablon:Period end İfade hatası: Tanınmayan "[" noktalama karakteri. milyon yıl önce, over 50% of today's angiosperm orders had evolved, and the clade accounted for 70% of global species.[86] It was around this time that flowering trees became dominant over conifers.[21]:498
The features of the basal "ANA" groups suggest that angiosperms originated in dark, damp, frequently disturbed areas.[87] It appears that the angiosperms remained constrained to such habitats throughout the Cretaceous – occupying the niche of small herbs early in the successional series.[86] This may have restricted their initial significance, but given them the flexibility that accounted for the rapidity of their later diversifications in other habitats.[87]
Şablon:Anthophyta Some propose that the Angiosperms arose from an unknown Seed Fern, Pteridophyte, and view Cycads as living Seed Ferns with both Seed-Bearing and sterile leaves (Cycas revoluta)[68]
In August 2017, scientists presented a detailed description and 3D image of a reconstruction of possibly the first flower that lived about 140 million years ago.[88][89]
Origins of the flower[değiştir | kaynağı değiştir]
The family Amborellaceae is regarded as being the sister clade to all other living flowering plants. A draft genome of Amborella trichopoda was published in December, 2013.[90] By comparing its genome with those of all other living flowering plants, it will be possible to work out the most likely characteristics of the ancestor of A. trichopoda and all other flowering plants, i.e. the ancestral flowering plant.[91]
It seems that on the level of the organ, the leaf may be the ancestor of the flower, or at least some floral organs. When some crucial genes involved in flower development are mutated, clusters of leaf-like structures arise in place of flowers. Thus, sometime in history, the developmental program leading to formation of a leaf must have been altered to generate a flower. There probably also exists an overall robust framework within which the floral diversity has been generated. An example of that is a gene called LEAFY (LFY), which is involved in flower development in Arabidopsis thaliana. The homologs of this gene are found in angiosperms as diverse as tomato, snapdragon, pea, maize and even gymnosperms. Expression of Arabidopsis thaliana LFY in distant plants like poplar and citrus also results in flower-production in these plants. The LFY gene regulates the expression of some genes belonging to the MADS-box family. These genes, in turn, act as direct controllers of flower development.[kaynak belirtilmeli]
Evolution of the MADS-box family[değiştir | kaynağı değiştir]
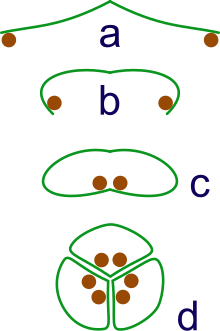
The members of the MADS-box family of transcription factors play a very important and evolutionarily conserved role in flower development. According to the ABC Model of flower development, three zones — A, B and C — are generated within the developing flower primordium, by the action of some transcription factors, that are members of the MADS-box family. Among these, the functions of the B and C domain genes have been evolutionarily more conserved than the A domain gene. Many of these genes have arisen through gene duplications of ancestral members of this family. Quite a few of them show redundant functions.
The evolution of the MADS-box family has been extensively studied. These genes are present even in pteridophytes, but the spread and diversity is many times higher in angiosperms.[92] There appears to be quite a bit of pattern into how this family has evolved. Consider the evolution of the C-region gene AGAMOUS (AG). It is expressed in today's flowers in the stamens, and the carpel, which are reproductive organs. Its ancestor in gymnosperms also has the same expression pattern. Here, it is expressed in the strobili, an organ that produces pollen or ovules.[93] Similarly, the B-genes' (AP3 and PI) ancestors are expressed only in the male organs in gymnosperms. Their descendants in the modern angiosperms also are expressed only in the stamens, the male reproductive organ. Thus, the same, then-existing components were used by the plants in a novel manner to generate the first flower. This is a recurring pattern in evolution.
Factors influencing floral diversity[değiştir | kaynağı değiştir]
There is enormous variation in floral structure in plants, typically due to changes in the MADS-box genes and their expression pattern. For example, grasses possess unique floral structures. The carpels and stamens are surrounded by scale-like lodicules and two bracts, the lemma and the palea, but genetic evidence and morphology suggest that lodicules are homologous to eudicot petals.[94] The palea and lemma may be homologous to sepals in other groups, or may be unique grass structures.[kaynak belirtilmeli]
Another example is that of Linaria vulgaris, which has two kinds of flower symmetries-radial and bilateral. These symmetries are due to epigenetic changes in just one gene called CYCLOIDEA.[79]

Arabidopsis thaliana has a gene called AGAMOUS that plays an important role in defining how many petals and sepals and other organs are generated. Mutations in this gene give rise to the floral meristem obtaining an indeterminate fate, and proliferation of floral organs in double-flowered forms of roses, carnations and morning glory. These phenotypes have been selected by horticulturists for their increased number of petals.[95] Several studies on diverse plants like petunia, tomato, Impatiens, maize etc. have suggested that the enormous diversity of flowers is a result of small changes in genes controlling their development.[96]
The Floral Genome Project confirmed that the ABC Model of flower development is not conserved across all angiosperms. Sometimes expression domains change, as in the case of many monocots, and also in some basal angiosperms like Amborella. Different models of flower development like the Fading boundaries model, or the Overlapping-boundaries model which propose non-rigid domains of expression, may explain these architectures.[97] There is a possibility that from the basal to the modern angiosperms, the domains of floral architecture have become more and more fixed through evolution.
Flowering time[değiştir | kaynağı değiştir]
Another floral feature that has been a subject of natural selection is flowering time. Some plants flower early in their life cycle, others require a period of vernalization before flowering. This outcome is based on factors like temperature, light intensity, presence of pollinators and other environmental signals: genes like CONSTANS (CO), Flowering Locus C (FLC) and FRIGIDA regulate integration of environmental signals into the pathway for flower development. Variations in these loci have been associated with flowering time variations between plants. For example, Arabidopsis thaliana ecotypes that grow in the cold, temperate regions require prolonged vernalization before they flower, while the tropical varieties, and the most common lab strains, don't. This variation is due to mutations in the FLC and FRIGIDA genes, rendering them non-functional.[98]
Many of the genes involved in this process are conserved across all the plants studied. Sometimes though, despite genetic conservation, the mechanism of action turns out to be different. For example, rice is a short-day plant, while Arabidopsis thaliana is a long-day plant. Both plants have the proteins CO and FLOWERING LOCUS T (FT), but, in Arabidopsis thaliana, CO enhances FT production, while in rice, the CO homolog represses FT production, resulting in completely opposite downstream effects.[99]
Theories of flower evolution[değiştir | kaynağı değiştir]
The Anthophyte theory was based on the observation that a gymnospermic group Gnetales has a flower-like ovule. It has partially developed vessels as found in the angiosperms, and the megasporangium is covered by three envelopes, like the ovary structure of angiosperm flowers. However, many other lines of evidence show that Gnetales is not related to angiosperms.[83]
The Mostly Male theory has a more genetic basis. Proponents of this theory point out that the gymnosperms have two very similar copies of the gene LFY, while angiosperms just have one. Molecular clock analysis has shown that the other LFY paralog was lost in angiosperms around the same time as flower fossils become abundant, suggesting that this event might have led to floral evolution.[100] According to this theory, loss of one of the LFY paralog led to flowers that were more male, with the ovules being expressed ectopically. These ovules initially performed the function of attracting pollinators, but sometime later, may have been integrated into the core flower.
Mechanisms and players in evolution of plant morphology[değiştir | kaynağı değiştir]

While environmental factors are significantly responsible for evolutionary change, they act merely as agents for natural selection. Change is inherently brought about via phenomena at the genetic level: mutations, chromosomal rearrangements, and epigenetic changes. While the general types of mutations hold true across the living world, in plants, some other mechanisms have been implicated as highly significant.
Genome doubling is a relatively common occurrence in plant evolution and results in polyploidy, which is consequently a common feature in plants. It is estimated that at least half (and probably all) plants have seen genome doubling in their history. Genome doubling entails gene duplication, thus generating functional redundancy in most genes. The duplicated genes may attain new function, either by changes in expression pattern or changes in activity. Polyploidy and gene duplication are believed to be among the most powerful forces in evolution of plant form; though it is not known why genome doubling is such a frequent process in plants. One probable reason is the production of large amounts of secondary metabolites in plant cells. Some of them might interfere in the normal process of chromosomal segregation, causing genome duplication.

In recent times, plants have been shown to possess significant microRNA families, which are conserved across many plant lineages. In comparison to animals, while the number of plant miRNA families are lesser than animals, the size of each family is much larger. The miRNA genes are also much more spread out in the genome than those in animals, where they are more clustered. It has been proposed that these miRNA families have expanded by duplications of chromosomal regions.[101] Many miRNA genes involved in regulation of plant development have been found to be quite conserved between plants studied.
Domestication of plants like maize, rice, barley, wheat etc. has also been a significant driving force in their evolution. Research concerning the origin of maize has found that it is a domesticated derivative of a wild plant from Mexico called teosinte. Teosinte belongs to the genus Zea, just as maize, but bears very small inflorescence, 5–10 hard cobs and a highly branched and spread out stem.

Crosses between a particular teosinte variety and maize yields fertile offspring that are intermediate in phenotype between maize and teosinte. QTL analysis has also revealed some loci that, when mutated in maize, yield a teosinte-like stem or teosinte-like cobs. Molecular clock analysis of these genes estimates their origins to some 9,000 years ago, well in accordance with other records of maize domestication. It is believed that a small group of farmers must have selected some maize-like natural mutant of teosinte some 9,000 years ago in Mexico, and subjected it to continuous selection to yield the familiar maize plant of today.[102]
The edible cauliflower is a domesticated version of the wild plant Brassica oleracea, which does not possess the dense undifferentiated inflorescence, called the curd, that cauliflower possesses.

Cauliflower possesses a single mutation in a gene called CAL, controlling meristem differentiation into inflorescence. This causes the cells at the floral meristem to gain an undifferentiated identity and, instead of growing into a flower, they grow into a dense mass of inflorescence meristem cells in arrested development.[103] This mutation has been selected through domestication since at least the time of the Greek empire.
Kaynakça[değiştir | kaynağı değiştir]
- ^ T. Cavalier Smith 2007, Evolution and relationships of algae major branches of the tree of life. from: Unravelling the algae, by Brodie & Lewis. CRC Press
- ^ Ševčíková, Tereza; ve diğerleri. (2015). "Updating algal evolutionary relationships through plastid genome sequencing". Scientific Reports. Cilt 5. s. 10134. Bibcode:2015NatSR...510134S. doi:10.1038/srep10134. PMC 4603697 $2. PMID 26017773.
- ^ Theodor Cole & Hartmut Hilger 2013 Bryophyte Phylogeny 23 Kasım 2015 tarihinde Wayback Machine sitesinde arşivlendi.
- ^ Theodor Cole & Hartmut Hilger 2013 Trachaeophyte Phylogeny 4 Mart 2016 tarihinde Wayback Machine sitesinde arşivlendi.
- ^ Theodor Cole & Hartmut Hilger 2015 Angiosperm Phylogeny, Flowering Plant Systematics. Freie Universität Berlin
- ^ Stewart, W.N.; Rothwell, G.W. (1993). Paleobotany and the evolution of plants. 2. Cambridge University Press. ISBN 978-0-521-23315-6.
- ^ Strother, Paul K.; Battison, Leila; Brasier, Martin D.; Wellman, Charles H. (2011). "Earth's earliest non-marine eukaryotes". Nature. 473 (7348). ss. 505–509. Bibcode:2011Natur.473..505S. doi:10.1038/nature09943. PMID 21490597.
- ^ Knauth, L. Paul; Kennedy, Martin J. (2009). "The late Precambrian greening of the Earth". Nature. 460 (7256). ss. 728–732. Bibcode:2009Natur.460..728K. doi:10.1038/nature08213. PMID 19587681.
- ^ Rothwell, G. W.; Scheckler, S. E.; Gillespie, W. H. (1989). "Elkinsia gen. nov., a Late Devonian gymnosperm with cupulate ovules". Botanical Gazette. 150 (2). ss. 170–189. doi:10.1086/337763.
- ^ a b Kenrick, Paul (2001). "Turning over a new leaf". Nature. 410 (6826). ss. 309–310. Bibcode:2001Natur.410..309K. doi:10.1038/35066649. PMID 11268183.
- ^ a b c Beerling D.; Osborne, C. P.; Chaloner, W. G. (2001). "Evolution of leaf-form in land plants linked to atmospheric CO2 decline in the Late Palaeozoic era" (PDF). Nature. 410 (6826). ss. 352–354. doi:10.1038/35066546. PMID 11268207.
- ^ Crane, P.R.; Kenrick, P. (1997). "Diverted development of reproductive organs: A source of morphological innovation in land plants". Plant Systematics and Evolution. 206 (1). ss. 161–174. doi:10.1007/BF00987946.
- ^ Zimmermann, W. (1959). Die Phylogenie der Pflanzen. 2. Stuttgart: Gustav Fischer Verlag.
- ^ Piazza, P.; ve diğerleri. (2005). "Evolution of leaf developmental mechanisms". New Phytol. 167 (3). ss. 693–710. doi:10.1111/j.1469-8137.2005.01466.x. PMID 16101907.
- ^ Hagemann, W. 1976. Sind Farne Kormophyten? Eine Alternative zur Telomtheorie. Plant Systematics and Evolution 124: 251–277.
- ^ Hagemann, W (1999). "Towards an organismic concept of land plants: the marginal blastozone and the development of the vegetation body of selected frondose gametophytes of liverworts and ferns". Plant Systematics and Evolution. 216 (1–2). ss. 81–133. doi:10.1007/bf00985102.
- ^ Sattler, R (1992). "Process morphology: structural dynamics in development and evolution". Canadian Journal of Botany. 70 (4). ss. 708–714. doi:10.1139/b92-091.
- ^ Sattler, R. 1998. On the origin of symmetry, branching and phyllotaxis in land plants. In: R.V. Jean and D. Barabé (eds) Symmetry in Plants. World Scientific, Singapore, pp. 775-793.
- ^ James, P.J. (2009). "'Tree and Leaf': A different angle". The Linnean. Cilt 25. s. 17.
- ^ "A perspective on the CO2 theory of early leaf evolution". 29 Haziran 2011 tarihinde kaynağından arşivlendi. Erişim tarihi: 8 Mart 2011.
- ^ a b c d e f g h i Kaynak hatası: Geçersiz
<ref>etiketi;StewartRothwell2isimli refler için metin sağlanmadı (Bkz: Kaynak gösterme) - ^ Kotyk, ME; Basinger, JF; Gensel, PG; de Freitas, TA (2002). "Morphologically complex plant macrofossils from the late Silurian of arctic Canada". American Journal of Botany. 89 (6). ss. 1004–1013. doi:10.3732/ajb.89.6.1004. PMID 21665700.
- ^ Rickards, R.B. (2000). "The age of the earliest club mosses: the Silurian Baragwanathia flora in Victoria, Australia" (abstract). Geological Magazine. 137 (2). ss. 207–209. Bibcode:2000GeoM..137..207R. doi:10.1017/S0016756800003800. Erişim tarihi: 25 Ekim 2007.
- ^ a b c d e Kaplan, D.R. (2001). "The Science of Plant Morphology: Definition, History, and Role in Modern Biology". American Journal of Botany. 88 (10). ss. 1711–1741. doi:10.2307/3558347. JSTOR 3558347. PMID 21669604.
- ^ Taylor, T.N.; Hass, H.; Kerp, H.; Krings, M.; Hanlin, R.T. (2005). "Perithecial ascomycetes from the 400 million year old Rhynie chert: an example of ancestral polymorphism". Mycologia. 97 (1). ss. 269–285. doi:10.3852/mycologia.97.1.269. hdl:1808/16786. PMID 16389979. Geçersiz
|hdl-access=free(yardım) - ^ a b c Boyce, C.K.; Knoll, A.H. (2002). "Evolution of developmental potential and the multiple independent origins of leaves in Paleozoic vascular plants". Paleobiology. 28 (1). ss. 70–100. doi:10.1666/0094-8373(2002)028<0070:EODPAT>2.0.CO;2. ISSN 0094-8373.
- ^ Hao, S.; Beck, C.B.; Deming, W. (2003). "Structure of the Earliest Leaves: Adaptations to High Concentrations of Atmospheric Şablon:Co2". International Journal of Plant Sciences. 164 (1). ss. 71–75. doi:10.1086/344557.
- ^ Berner, R.A.; Kothavala, Z. (2001). "Geocarb III: A Revised Model of Atmospheric [[:Şablon:Co2]] over Phanerozoic Time". American Journal of Science. 301 (2). ss. 182–204. Bibcode:2001AmJS..301..182B. CiteSeerX 10.1.1.393.582 $2. doi:10.2475/ajs.301.2.182. 29 Ocak 2009 tarihinde kaynağından (abstract) arşivlendi. Erişim tarihi: 7 Nisan 2008. URL–vikibağı karışıklığı (yardım)
- ^ Taylor, T.N.; Taylor, E.L. (1993). "The biology and evolution of fossil plants".
- ^ Shellito, C.J.; Sloan, L.C. (2006). "Reconstructing a lost Eocene paradise: Part I. Simulating the change in global floral distribution at the initial Eocene thermal maximum". Global and Planetary Change. 50 (1–2). ss. 1–17. Bibcode:2006GPC....50....1S. doi:10.1016/j.gloplacha.2005.08.001.
- ^ Aerts, R. (1995). "The advantages of being evergreen". Trends in Ecology & Evolution. 10 (10). ss. 402–407. doi:10.1016/S0169-5347(00)89156-9. PMID 21237084.
- ^ Labandeira, C.C.; Dilcher, D.L.; Davis, D.R.; Wagner, D.L. (1994). "Ninety-seven million years of angiosperm-insect association: paleobiological insights into the meaning of coevolution". Proceedings of the National Academy of Sciences of the United States of America. 91 (25). ss. 12278–12282. Bibcode:1994PNAS...9112278L. doi:10.1073/pnas.91.25.12278. PMC 45420 $2. PMID 11607501.
- ^ Brown V; ve diğerleri. (1991). "Herbivory and the Evolution of Leaf Size and Shape". Philosophical Transactions of the Royal Society B. 333 (1267). ss. 265–272. doi:10.1098/rstb.1991.0076.
- ^ Harrison C. J.; ve diğerleri. (2005). "Independent recruitment of a conserved developmental mechanism during leaf evolution". Nature. 434 (7032). ss. 509–514. Bibcode:2005Natur.434..509H. doi:10.1038/nature03410. PMID 15791256.
- ^ Jackson D.; Hake S. (1999). "Control of Phyllotaxy in Maize by the ABPHYL1 Gene". Development. 126 (2). ss. 315–323. PMID 9847245.
- ^ Cronk Q. (2001). "Plant evolution and development in a post-genomic context". Nature Reviews Genetics. 2 (8). ss. 607–619. doi:10.1038/35084556. PMID 11483985.
- ^ Tattersall; ve diğerleri. (2005). "The Mutant crispa Reveals Multiple Roles for PHANTASTICA in Pea Compound Leaf Development". Plant Cell. 17 (4). ss. 1046–1060. doi:10.1105/tpc.104.029447. PMC 1087985 $2. PMID 15749758.
- ^ Bharathan, Geeta; Sinha, Neelima Roy (Dec 2001). "The Regulation of Compound Leaf Development". Plant Physiol. 127 (4). ss. 1533–1538. doi:10.1104/pp. 010867. PMC 1540187 $2. PMID 11743098.
- ^ Nath, U; Crawford, BC; Carpenter, R; ve diğerleri. (2003). "Genetic Control of Surface Curvature". Science. 299 (5611). ss. 1404–1407. CiteSeerX 10.1.1.625.1791 $2. doi:10.1126/science.1079354. PMID 12610308.
- ^ Li, Chao; Zhang, Baohong (February 2016). "MicroRNAs in Control of Plant Development". Journal of Cellular Physiology. 231 (2). ss. 303–13. doi:10.1002/jcp.25125. PMID 26248304.
- ^ Mecchia, MA; Debernardi, JM; Rodriguez, RE; Schommer, C; Palatnik, JF (January 2013). "MicroRNA miR396 and RDR6 synergistically regulate leaf development". Mechanisms of Development. 130 (1). ss. 2–13. doi:10.1016/j.mod.2012.07.005. PMID 22889666. Geçersiz
|doi-access=free(yardım) - ^ de Souza, Rocheli; Ambrosini, Adriana; Passaglia, Luciane M.P. (December 2015). "Plant growth-promoting bacteria as inoculants in agricultural soils". Genetics and Molecular Biology. 38 (4). ss. 401–19. doi:10.1590/S1415-475738420150053. PMC 4763327 $2. PMID 26537605.
- ^ Mora, C.I.; Driese, S.G.; Colarusso, L.A. (1996). "Middle to Late Paleozoic Atmospheric Şablon:Co2 Levels from Soil Carbonate and Organic Matter". Science. 271 (5252). ss. 1105–1107. Bibcode:1996Sci...271.1105M. doi:10.1126/science.271.5252.1105.
- ^ Berner, R.A. (1994). "GEOCARB II: A revised model of atmospheric Şablon:Co2 over Phanerozoic time". Am. J. Sci. 294 (1). ss. 56–91. Bibcode:1994AmJS..294...56B. doi:10.2475/ajs.294.1.56.
- ^ a b Algeo, T.J.; Berner, R.A.; Maynard, J.B.; Scheckler, S.E.; Archives, G.S.A.T. (1995). "Late Devonian Oceanic Anoxic Events and Biotic Crises: "Rooted" in the Evolution of Vascular Land Plants?". GSA Today. 5 (3).
- ^ Retallack, G. J. (1986). Wright, V. P. (Ed.). Paleosols: their Recognition and Interpretation. Oxford: Blackwell.
- ^ a b c d e f g h i j k Raven, J.A.; Edwards, D. (2001). "Roots: evolutionary origins and biogeochemical significance". Journal of Experimental Botany. 52 (90001). ss. 381–401. doi:10.1093/jexbot/52.suppl_1.381. PMID 11326045. Geçersiz
|doi-access=free(yardım) - ^ "Tracheophyta - 2". Paleos - Life Through Deep Time.
- ^ Richard M. Bateman, Peter R. Crane, William A. DiMichele, Paul R. Kenrick, Nick P. Rowe, Thomas Speck, and William E. Stein (1998). "EARLY EVOLUTION OF LAND PLANTS: Phylogeny, Physiology, and Ecology of the Primary Terrestrial Radiation". Annual Review of Ecology and Systematics. Cilt 29. ss. 263–292. doi:10.1146/annurev.ecolsys.29.1.263.
- ^ Hetherington, Alexander J; Dolan, Liam (2017). "Bilaterally symmetric axes with rhizoids composed the rooting structure of the common ancestor of vascular plants". Philosophical Transactions of the Royal Society B: Biological Sciences. 373 (1739). s. 20170042. doi:10.1098/rstb.2017.0042. PMC 5745339 $2. PMID 29254968.
- ^ a b c d e f g h i Algeo, T.J.; Scheckler, S.E. (1998). "Terrestrial-marine teleconnections in the Devonian: links between the evolution of land plants, weathering processes, and marine anoxic events". Philosophical Transactions of the Royal Society B. 353 (1365). ss. 113–130. doi:10.1098/rstb.1998.0195. PMC 1692181 $2.
- ^ Hetherington, Alexander J.; Dolan, Liam (2018). "Stepwise and independent origins of roots among land plants". Nature. 561 (7722). ss. 235–238. Bibcode:2018Natur.561..235H. doi:10.1038/s41586-018-0445-z. PMC 6175059 $2. PMID 30135586.
- ^ Coudert, Yoan; Anne Dievart; Gaetan Droc; Pascal Gantet (2012). "ASL/LBD Phylogeny Suggests that Genetic Mechanisms of Root Initiation Downstream of Auxin Are Distinct in Lycophytes and Euphyllophytes". Molecular Biology and Evolution. 30 (3). ss. 569–72. doi:10.1093/molbev/mss250. ISSN 0737-4038. PMID 23112232. Geçersiz
|doi-access=free(yardım) - ^ Kaynak hatası: Geçersiz
<ref>etiketi;KCbookisimli refler için metin sağlanmadı (Bkz: Kaynak gösterme) - ^ a b Kenrick, P.; Crane, P.R. (1997). "The origin and early evolution of plants on land". Nature. 389 (6646). ss. 33–39. Bibcode:1997Natur.389...33K. doi:10.1038/37918.
- ^ Alain Pierret, Jean-Luc Maeght, Corentin Clément, Jean-Pierre Montoroi, Christian Hartmann, Santimaitree Gonkhamdee (July 2016). "Understanding deep roots and their functions in ecosystems: an advocacy for more unconventional research". Annals of Botany. 118 (4). ss. 621–635. doi:10.1093/aob/mcw130. PMC 5055635 $2. PMID 27390351.
- ^ Gerrienne, Philippe; Gensel, Patricia G.; Strullu-Derrien, Christine; Lardeux, Hubert; Steemans, Philippe; Prestianni, Cyrille (2011). "A simple type of wood in two early Devonian plants". Science. 333 (6044). s. 837. Bibcode:2011Sci...333..837G. doi:10.1126/science.1208882. PMID 21836008.
- ^ Kinver, Mark (16 Ağustos 2011). "'Early wood' samples reshape plant history". BBC News.
- ^ Stein, W.E.; Mannolini, F.; Hernick, L.V.; Landing, E.; Berry, C.M. (2007). "Giant cladoxylopsid trees resolve the enigma of the Earth's earliest forest stumps at Gilboa". Nature. 446 (7138). ss. 904–7. Bibcode:2007Natur.446..904S. doi:10.1038/nature05705. PMID 17443185.
- ^ Retallack, G.J.; Catt, J.A.; Chaloner, W.G. (1985). "Fossil Soils as Grounds for Interpreting the Advent of Large Plants and Animals on Land [and Discussion]". Philosophical Transactions of the Royal Society B. 309 (1138). ss. 105–142. Bibcode:1985RSPTB.309..105R. doi:10.1098/rstb.1985.0074. JSTOR 2396355. Geçersiz
|doi-access=free(yardım) - ^ Dannenhoffer, J.M.; Bonamo, P.M. (1989). "Rellimia thomsonii from the Givetian of New York: Secondary Growth in Three Orders of Branching". American Journal of Botany. 76 (9). ss. 1312–1325. doi:10.2307/2444557. JSTOR 2444557.
- ^ Davis, P; Kenrick, P. (2004). Fossil Plants. Smithsonian Books, Washington D.C. ISBN 978-1-58834-156-3.
- ^ Donoghue, M.J. (2005). "Key innovations, convergence, and success: macroevolutionary lessons from plant phylogeny" (abstract). Paleobiology. 31 (2). ss. 77–93. doi:10.1666/0094-8373(2005)031[0077:KICASM]2.0.CO;2. ISSN 0094-8373. Erişim tarihi: 7 Nisan 2008.
- ^ a b c Bowe, L.M.; Coat, G.; Depamphilis, C.W. (2000). "Phylogeny of seed plants based on all three genomic compartments: Extant gymnosperms are monophyletic and Gnetales' closest relatives are conifers". Proceedings of the National Academy of Sciences. 97 (8). ss. 4092–7. Bibcode:2000PNAS...97.4092B. doi:10.1073/pnas.97.8.4092. PMC 18159 $2. PMID 10760278.
- ^ a b c Chaw, S.M.; Parkinson, C.L.; Cheng, Y.; Vincent, T.M.; Palmer, J.D. (2000). "Seed plant phylogeny inferred from all three plant genomes: Monophyly of extant gymnosperms and origin of Gnetales from conifers". Proceedings of the National Academy of Sciences. 97 (8). ss. 4086–91. Bibcode:2000PNAS...97.4086C. doi:10.1073/pnas.97.8.4086. PMC 18157 $2. PMID 10760277.
- ^ a b c Soltis, D.E.; Soltis, P.S.; Zanis, M.J. (2002). "Phylogeny of seed plants based on evidence from eight genes". American Journal of Botany. 89 (10). ss. 1670–81. doi:10.3732/ajb.89.10.1670. PMID 21665594. 12 Nisan 2008 tarihinde kaynağından (abstract) arşivlendi. Erişim tarihi: 8 Nisan 2008.
- ^ Friis, E.M.; Pedersen, K.R.; Crane, P.R. (2006). "Cretaceous angiosperm flowers: Innovation and evolution in plant reproduction". Palaeogeography, Palaeoclimatology, Palaeoecology. 232 (2–4). ss. 251–293. Bibcode:2006PPP...232..251F. doi:10.1016/j.palaeo.2005.07.006.
- ^ a b Hilton, J.; Bateman, R.M. (2006). "Pteridosperms are the backbone of seed-plant phylogeny". The Journal of the Torrey Botanical Society. 133 (1). ss. 119–168. doi:10.3159/1095-5674(2006)133[119:PATBOS]2.0.CO;2. ISSN 1095-5674.
- ^ a b Bateman, R.M.; Hilton, J.; Rudall, P.J. (2006). "Morphological and molecular phylogenetic context of the angiosperms: contrasting the 'top-down' and 'bottom-up' approaches used to infer the likely characteristics of the first flowers". Journal of Experimental Botany. 57 (13). ss. 3471–503. doi:10.1093/jxb/erl128. PMID 17056677. Geçersiz
|doi-access=free(yardım) - ^ a b c d Frohlich, M.W.; Chase, M.W. (2007). "After a dozen years of progress the origin of angiosperms is still a great mystery". Nature. 450 (7173). ss. 1184–9. Bibcode:2007Natur.450.1184F. doi:10.1038/nature06393. PMID 18097399.
- ^ "Science Magazine". Runcaria, a Middle Devonian Seed Plant Precursor. American Association for the Advancement of Science. 2011. Erişim tarihi: 22 Mart 2011.
- ^ a b c Mapes, G.; Rothwell, G.W.; Haworth, M.T. (1989). "Evolution of seed dormancy". Nature. 337 (6208). ss. 645–646. Bibcode:1989Natur.337..645M. doi:10.1038/337645a0.
- ^ Bawa, KS; Webb, CJ (1984). "Flower, fruit and seed abortion in tropical forest trees. Implications for the evolution of paternal and maternal reproductive patterns". American Journal of Botany. 71 (5). ss. 736–751. doi:10.2307/2443371. JSTOR 2443371.
- ^ Cheah KS, Osborne DJ (April 1978). "DNA lesions occur with loss of viability in embryos of ageing rye seed". Nature. 272 (5654). ss. 593–9. Bibcode:1978Natur.272..593C. doi:10.1038/272593a0. PMID 19213149.
- ^ Koppen G, Verschaeve L (2001). "The alkaline single-cell gel electrophoresis/comet assay: a way to study DNA repair in radicle cells of germinating Vicia faba". Folia Biol. (Praha). 47 (2). ss. 50–4. PMID 11321247.
- ^ Bray CM, West CE (December 2005). "DNA repair mechanisms in plants: crucial sensors and effectors for the maintenance of genome integrity". New Phytol. 168 (3). ss. 511–28. doi:10.1111/j.1469-8137.2005.01548.x. PMID 16313635.
- ^ Bernstein C, Bernstein H. (1991) Aging, Sex, and DNA Repair. Academic Press, San Diego. 0120928604 978-0120928606
- ^ Feild, T. S.; Brodribb, T. J.; Iglesias, A.; Chatelet, D. S.; Baresch, A.; Upchurch, G. R.; Gomez, B.; Mohr, B. A. R.; Coiffard, C.; Kvacek, J.; Jaramillo, C. (2011). "Fossil evidence for Cretaceous escalation in angiosperm leaf vein evolution". Proceedings of the National Academy of Sciences. 108 (20). ss. 8363–8366. Bibcode:2011PNAS..108.8363F. doi:10.1073/pnas.1014456108. PMC 3100944 $2. PMID 21536892.
- ^ a b Lawton-Rauh, A.; Alvarez-Buylla, E.R.; Purugganan, M.D. (2000). "Molecular evolution of flower development". Trends in Ecology and Evolution. 15 (4). ss. 144–149. doi:10.1016/S0169-5347(99)01816-9. PMID 10717683.
- ^ Chinese Academy of Sciences (18 Aralık 2018). "Flowers originated 50 million years earlier than previously thought". EurekAlert!. Erişim tarihi: 18 Aralık 2018.
- ^ Coiro, Mario; Doyle, James A.; Hilton, Jason (25 Ocak 2019). "How deep is the conflict between molecular and fossil evidence on the age of angiosperms?". New Phytologist (İngilizce). 223 (1). ss. 83–99. doi:10.1111/nph.15708. PMID 30681148. Geçersiz
|doi-access=free(yardım) - ^ Nam, J.; Depamphilis, CW; Ma, H; Nei, M (2003). "Antiquity and Evolution of the MADS-Box Gene Family Controlling Flower Development in Plants". Mol. Biol. Evol. 20 (9). ss. 1435–1447. doi:10.1093/molbev/msg152. PMID 12777513. Geçersiz
|doi-access=free(yardım) - ^ a b Kaynak hatası: Geçersiz
<ref>etiketi;Crepet2000isimli refler için metin sağlanmadı (Bkz: Kaynak gösterme) - ^ Sun, G.; Ji, Q.; Dilcher, D.L.; Zheng, S.; Nixon, K.C.; Wang, X. (2002). "Archaefructaceae, a New Basal Angiosperm Family". Science. 296 (5569). ss. 899–904. Bibcode:2002Sci...296..899S. doi:10.1126/science.1069439. PMID 11988572.
- ^ In fact, Archaefructus probably didn't bear true flowers: see
- Friis, E.M.; Doyle, J.A.; Endress, P.K.; Leng, Q. (2003). "Archaefructus—angiosperm precursor or specialized early angiosperm?". Trends in Plant Science. 8 (8). ss. 369–373. doi:10.1016/S1360-1385(03)00161-4. PMID 12927969.
- ^ a b Wing, S.L.; Boucher, L.D. (1998). "Ecological Aspects Of The Cretaceous Flowering Plant Radiation". Annual Review of Earth and Planetary Sciences. 26 (1). ss. 379–421. Bibcode:1998AREPS..26..379W. doi:10.1146/annurev.earth.26.1.379.
- ^ a b Feild, T.S.; Arens, N.C.; Doyle, J.A.; Dawson, T.E.; Donoghue, M.J. (2004). "Dark and disturbed: a new image of early angiosperm ecology" (abstract). Paleobiology. 30 (1). ss. 82–107. doi:10.1666/0094-8373(2004)030<0082:DADANI>2.0.CO;2. ISSN 0094-8373. Erişim tarihi: 8 Nisan 2008.
- ^ Gabbott, Prof Sarah (1 Ağustos 2017). "Did the first flower look like this?". BBC News. Erişim tarihi: 1 Ağustos 2017.
- ^ Sauquet, Hervé; ve diğerleri. (1 Ağustos 2017). "The ancestral flower of angiosperms and its early diversification". Nature Communications. Cilt 8. s. 16047. Bibcode:2017NatCo...816047S. doi:10.1038/ncomms16047. PMC 5543309 $2. PMID 28763051.
- ^ Amborella Genome Project (20 Dec 2013). "The Amborella Genome and the Evolution of Flowering Plants". Science. 342 (6165). s. 1241089. doi:10.1126/science.1241089. PMID 24357323. Erişim tarihi: 1 Şubat 2020.
- ^ Zuccolo, A.; ve diğerleri. (2011). "A physical map for the Amborella trichopoda genome sheds light on the evolution of angiosperm genome structure". Genome Biology. 12 (5). s. R48. doi:10.1186/gb-2011-12-5-r48. PMC 3219971 $2. PMID 21619600.
- ^ Medarg NG, Yanofsky M (March 2001). "Function and evolution of the plant MADS-box gene family". Nature Reviews Genetics. 2 (3). ss. 186–195. doi:10.1038/35056041. PMID 11256070.
- ^ Jager; ve diğerleri. (2003). "MADS-Box Genes in Ginkgo biloba and the Evolution of the AGAMOUS Family". Mol. Biol. Evol. 20 (5). ss. 842–854. doi:10.1093/molbev/msg089. PMID 12679535. Geçersiz
|doi-access=free(yardım) - ^ Translational Biology: From Arabidopsis Flowers to Grass Inflorescence Architecture. Beth E. Thompson* and Sarah Hake, 2009, Plant Physiology 149:38–45.
- ^ Kitahara K, Matsumoto S (2000). "Rose MADS-box genes 'MASAKO C1 and D1' homologous to class C floral identity genes". Plant Science. 151 (2). ss. 121–134. doi:10.1016/S0168-9452(99)00206-X. PMID 10808068.
- ^ Kater M; ve diğerleri. (1998). "Multiple AGAMOUS Homologs from Cucumber and Petunia Differ in Their Ability to Induce Reproductive Organ Fate". Plant Cell. 10 (2). ss. 171–182. doi:10.1105/tpc.10.2.171. PMC 143982 $2. PMID 9490741.
- ^ Soltis D; ve diğerleri. (2007). "The floral genome: an evolutionary history of gene duplication and shifting patterns of gene expression". Trends Plant Sci. 12 (8). ss. 358–367. doi:10.1016/j.tplants.2007.06.012. PMID 17658290.
- ^ Putterhill; ve diğerleri. (2004). "It's time to flower: the genetic control of flowering time". BioEssays. 26 (4). ss. 353–363. doi:10.1002/bies.20021. PMID 15057934. 16 Aralık 2012 tarihinde kaynağından arşivlendi.
- ^ Blazquez; ve diğerleri. (2001). "Flowering on time: genes that regulate the floral transition". EMBO Reports. 2 (12). ss. 1078–1082. doi:10.1093/embo-reports/kve254. PMC 1084172 $2. PMID 11743019.
- ^ Frohlich, Michael W.; Parker, David S. (2000). "The Mostly Male Theory of Flower Evolutionary Origins: from Genes to Fossils". Syst. Bot. 25 (2). ss. 155–170. doi:10.2307/2666635. JSTOR 2666635.
- ^ Li A, Mao L (2007). "Evolution of plant microRNA gene families". Cell Research. 17 (3). ss. 212–218. doi:10.1038/sj.cr.7310113. PMID 17130846. Geçersiz
|doi-access=free(yardım) - ^ Doebley J.F. (2004). "The genetics of maize evolution". Annu. Rev. Genet. 38 (1). ss. 37–59. doi:10.1146/annurev.genet.38.072902.092425. PMID 15568971.
- ^ Purugannan; ve diğerleri. (2000). "Variation and Selection at the CAULIFLOWER Floral Homeotic Gene Accompanying the Evolution of Domesticated Brassica olerace". Genetics. 155 (2). ss. 855–862. PMC 1461124 $2. PMID 10835404.


